![2+ p 2+ r
G(V, [Ca ],t) = ˉgm(V, t) z([Ca ],t) , (units: mV, μM, ms)](pub-purkinje-deschutter1-hh0x.png)
Related Documentation:
The channel conductance was determined by the product of voltage-dependent activation (m) and inactivation (h) gates, and for the Ca2+-activated channels a Ca2+-dependent activation gate (z)
![2+ p 2+ r
G(V, [Ca ],t) = ˉgm(V, t) z([Ca ],t) , (units: mV, μM, ms)](pub-purkinje-deschutter1-hh0x.png) | (1) |
Equations describing the voltage-dependent gates were described from the classic Hodgkin-Huxley [2] scheme
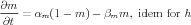 | (2) |
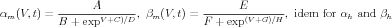 | (3) |
Activation rates for Ca2+-dependent gates were determined by a dissociation constant A and a time constant B
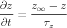 | (4) |
![-----1-----
z∞ = 1 + ---A---τz = B
[Ca2+]](pub-purkinje-deschutter1-hh4x.png) | (5) |
For the Ca2+ channels the Nernst potential [1] was computed continuously.
Rectification of Ca2+ channels was not modeled using the Goldman-Hodgkin-Katz
(GHK) equation [1] because dendritic membrane potentials in this study stayed
within a range where Ca2+ channels can be considered ohmic (i.e., below -20 mV;
Fig. 4.15 in [1] ). Using the simulation results from the final model, we estimate
that using the GHK equation with an appropriately scaled maximum conductance
( ) to compensate for differences in driving force would cause only small changes
in the amplitude of dendritic Ca2+ currents (mean difference 0.7 %, maximum
4.5 %).
) to compensate for differences in driving force would cause only small changes
in the amplitude of dendritic Ca2+ currents (mean difference 0.7 %, maximum
4.5 %).